
Individualized and person-centred physical activity for patients with non-functioning pituitary adenoma: study protocol for a randomized controlled trial Har passerat
Onsdag 15 maj 2024 14:30 - 15:13 Poster Arena
Rapportör: Anna SandbergSpår: Posters, Evaluation of Interventions
Poster can be found in location 111.
Individualized and person-centred physical activity
for patients with non-functioning pituitary adenoma:
study protocol for a randomized controlled trial
___________________________________________________________________________________________
Introduction
Patients with pituitary adenoma have reduced self-reported quality of life (QoL) and well-being (1,2), and an increased risk of developing cardiovascular diseases (3).
Person-centred care focuses on preserving health and wellbeing by acknowledging patients' resources, motivations and strengths, and strives to engage the patient in health-related decision-making (4). Physiotherapy outlined within a person-centred approach is effective for a broad range of disorders (5). The effect of increased physical activity in patients with pituitary adenoma is, however, yet unexplored.
Aim
To study the effects of person-centred physical activity on QoL, cardiorespiratory fitness, fatigue, self-efficacy, and cardiovascular risk profile in surgically treated patients with non-functioning pituitary adenoma. The main hypothesis is that increased physical activity improves QoL.
Methods
This is a prospective, randomized, controlled trial where patients with non-functioning pituitary adenoma, treated with pituitary surgery during the last ten years at the Sahlgrenska University Hospital in Gothenburg, Sweden, will be screened for eligibility. 120 participants will be randomly assigned to either intervention or standard care (Figure 1). The intervention group will receive an individualized and person-centred prescription of physical activity and exercise, described in figure 2. Intervention follow-up visits (in-person or digitally) will be scheduled at 4, 8 and 12 weeks by a physiotherapist. The study outcome measures are presented in Table 1 and will be assessed at baseline, six and twelve months by a blinded evaluator. This study is approved by the Swedish Ethical Review Authority (Dnr: 2023-02770-01).
Potential clinical benefits
If our hypothesis is correct, QoL in patients with non-functioning pituitary adenoma can be improved by individualized and person-centred physical activity and this, in turn, strengthens the patient’s confidence in managing self-care.
Authors declare that no corporate sponsors, shareholdings, patents, or other commercial interests are linked to the project.
Table 1. Outcome measurements, assessed at baseline, six and twelve months by blinded evaluator
Endpoint | Assessment objective | Specific test or questionnaire |
Primary endpoint | Health-related quality of life | SF-36 |
Secondary endpoints | Health status | EQ5D |
Physical activity | SGPALS, GIH-Sed and with an accelerometer | |
Physical fitness | RPE-scale | |
Cardiorespiratory fitness | Submaximal EKBLOM-BAK test | |
Muscle strength | Grip strength will be measured with JAMAR and muscle strength of the lower extremities with the chair stand test | |
Self-reported fatigue | MFI-20 | |
Self-reported self-efficacy | Self-Efficacy to Manage Chronic Disease Scale | |
Cardiovascular risk profile | Weight and waist circumference, Body composition, blood pressure, Lipids, Glucose metabolism and hs-CRP | |
Patients’ perceptions of person-centred care | GPCCQ |
Between-group differences will be calculated and analysed from baseline to 6 and 12 months. SF-36; short form 36, EQ5D; EuroQol-5D, SGPALS; Saltin-Grimby Physical Activity Level Scale, GIH-Sed; Single-Item Question for Assessment of Sedentary Behaviour, RPE-scale; Rating of Perceived Exertion Scale, MFI-20; Multidimensional Fatigue Inventory-20, GPCCQ; Generic Patient Perceptions of Person-centred Care Questionnaire
Figure 1. Study flow chart. 1 All participants will receive standard care, including recommendations on physical activity (6) and meetings with an endocrinologist at baseline and after 6 and 12 months. Health related issues and perceived symptoms are reviewed and, when needed, doses of hormonal replacement are adjusted during the visits. 2 Individualized and person-centred physical activity and exercise
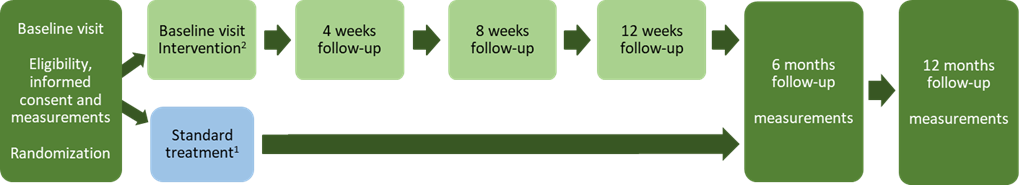
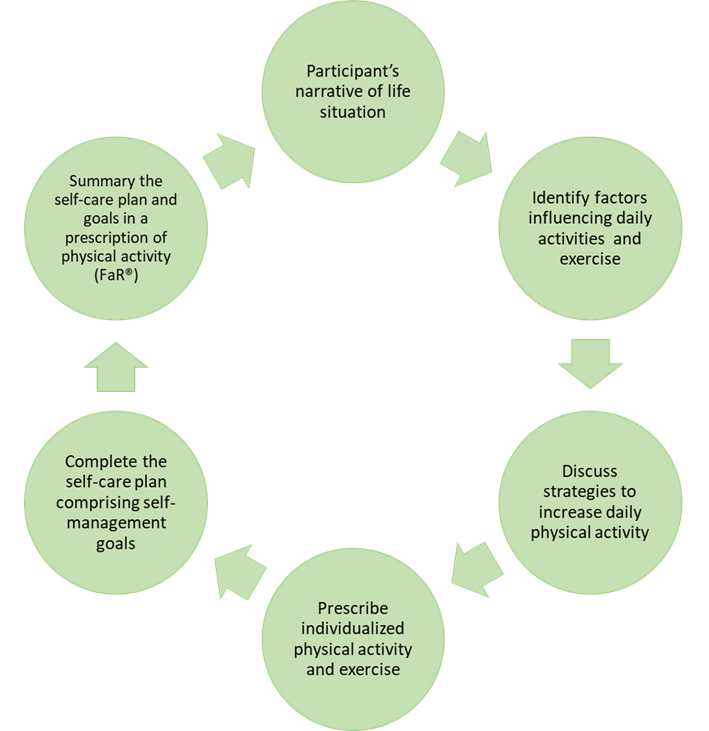
Figure 2. Description of the study intervention with examples of the interactive parts of the baseline meeting between patient, physiotherapist, and nurse.
References
- Andela CD, Lobatto DJ, Pereira AM, van Furth WR, Biermasz NR. How non-functioning pituitary adenomas can affect health-related quality of life: a conceptual model and literature review. Pituitary. 2018;21(2):208-16.
- Jakobsson Ung E, Olofsson AC, Bjorkman I, Hallen T, Olsson DS, Ragnarsson O, et al. The pre- and postoperative illness trajectory in patients with pituitary tumours. Endocr Connect. 2019;8(7):878-86.
- Esposito D, Johannsson G, Ragnarsson O. Chapter 9 - Endocrinological diagnosis and replacement therapy for hypopituitarism. In: Honegger J, Reincke M, Petersenn S, editors. Pituitary Tumors: Academic Press; 2021. p. 135-46.
- Ekman I, Swedberg K, Taft C, Lindseth A, Norberg A, Brink E, et al. Person-centered care--ready for prime time. Eur J Cardiovasc Nurs. 2011;10(4):248-51.
- Melin J, Nordin A, Feldthusen C, Danielsson L. Goal-setting in physiotherapy: exploring a person-centered perspective. Physiother Theory Pract. 2021;37(8):863-80
- WHO, 2020, https://www.who.int/publications/i/item/9789240015128
Seminarietyp
Poster
Konferens
GCPCC
Authors
Anna Sandberg, Victor Hantelius , Oskar Ragnarsson, Thomas Skoglund, Gudmundur Johannsson, Sofie Jakobsson
Föreläsare
Anna Sandberg Rapportör
Physioterapist, PhD